Research Insights
April 11, 2024
Propylene glycol - a specific biomarker for the assessment of e-cigarette exposure
Are you intrigued by the evolving landscape of tobacco products and the scientific quest to accurately monitor their use? Manifold tobacco and nicotine products have been evolving, bringing forth a myriad of nicotine-delivery systems that challenge traditional monitoring methods. Among these, electronic cigarettes have sparked conversations about their role in smoking cessation and the need for precise exposure assessment tools.
Exposure Specific to e-Cigarette Consumption
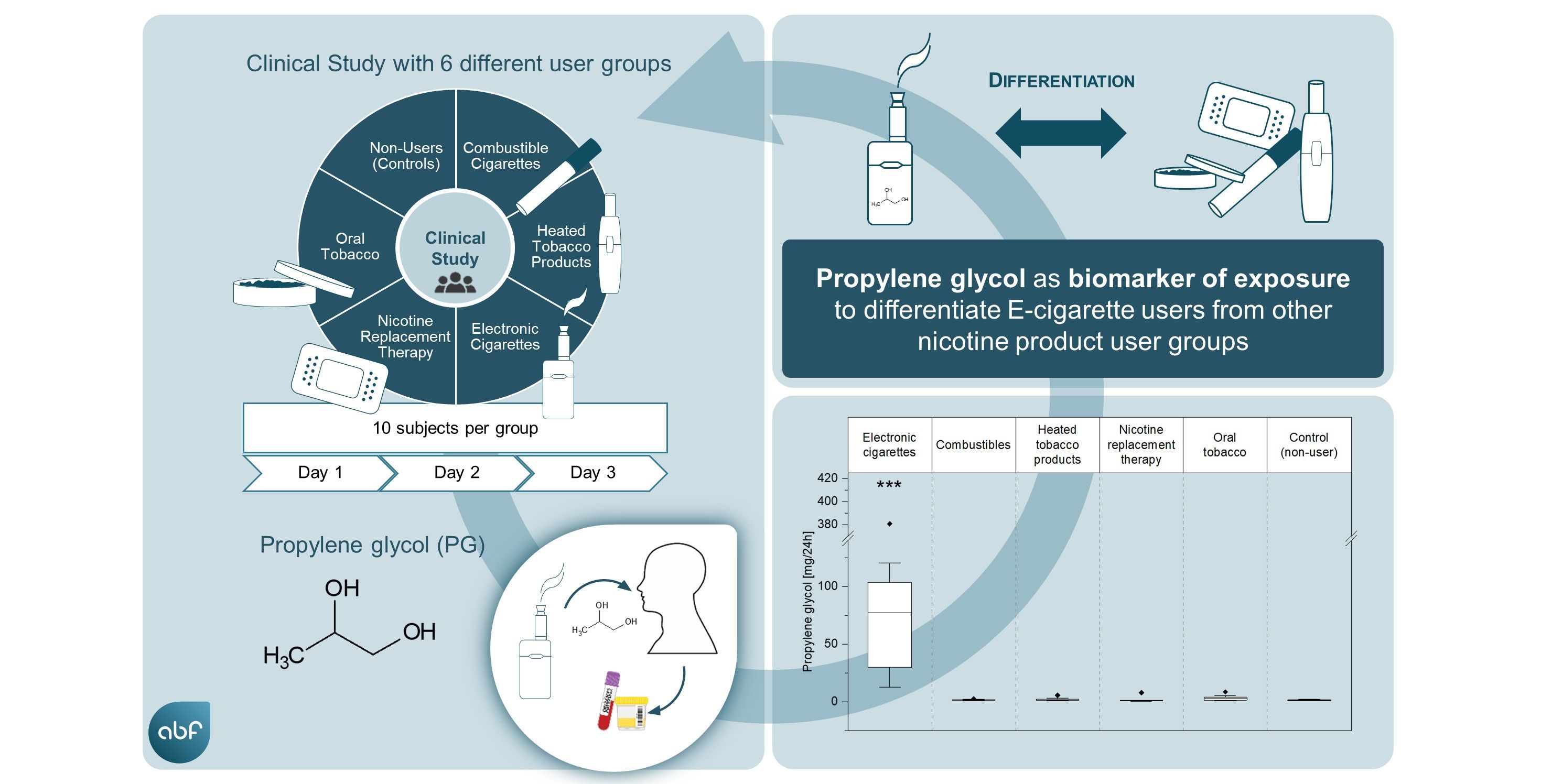
Our study addressed the potential use of biomarkers of exposure (BoE) specific to electronic cigarette (EC) use (vaping) for exposure assessment and compliance monitoring. We established a sensitive LC-MS/MS method for quantification of 1,2-propylene glycol (PG) and glycerol (G) in plasma and urine samples from users of different nicotine products, including combustible cigarettes (CC), ECs, heated tobacco products (HTP), oral tobacco (OT), and oral/dermal nicotine delivery products used for nicotine replacement therapy (NRT), and a control group of non-users (NU). The results showed significantly elevated PG levels in plasma and urine of EC users compared to all other user groups and the control group, suggesting PG's high specificity as a BoE for EC consumption. This finding supports the potential use of PG as a biomarker for monitoring EC use compliance in exposure assessments.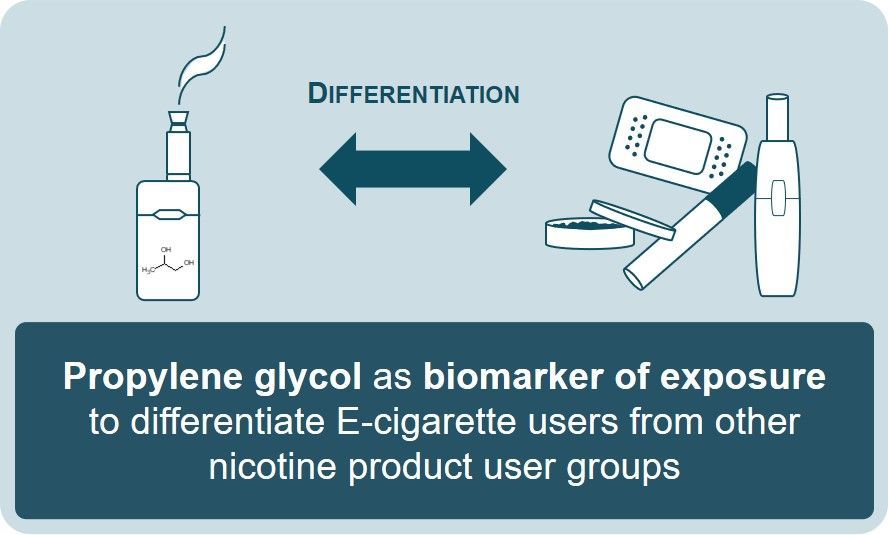
PG: A Biomarker with High Specificity for EC Use
This discovery is pivotal, as it suggests that PG can be used to monitor EC use compliance and assess exposure with high specificity. The study's controlled clinical approach clearly differentiated EC users from other nicotine product users based on PG levels, marking a significant step forward in the search for EC use specific biomarkers of exposure. Moreover, the research indicated a dose-response relationship between urinary PG excretion and the intensity of EC vaping. This finding opens the door for using PG as a reliable compliance marker in exposure assessments under real-life conditions.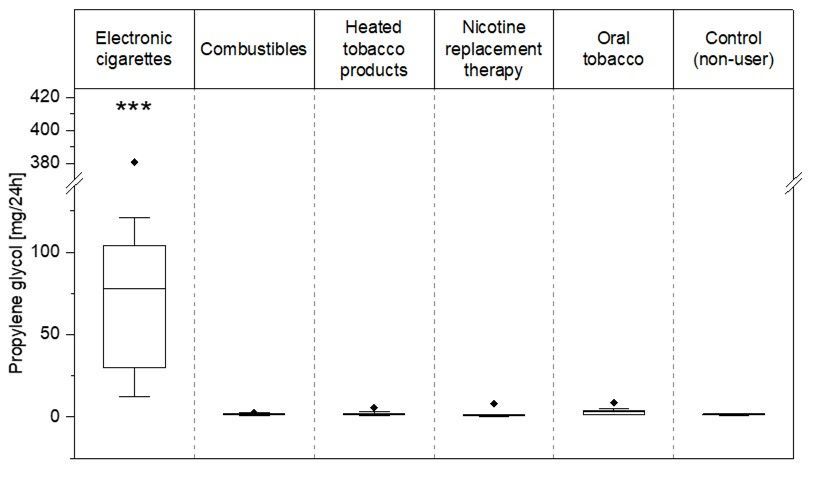
The Road Ahead: Larger Studies and Comprehensive Biomarker Combinations
While the results are promising, the study calls for larger-scale research to confirm the suitability of PG as a biomarker for EC use. It also suggests that a combination of biomarkers may offer a more nuanced understanding of exposure and its health implications.